WHAT IS AMMONIA GAS?
The New York Department of Health describes ammonia gas as a colorless, alkaline gas comprised of nitrogen and hydrogen (NH3) that has a strong odor, often associated with window cleaner. Ammonia is a natural, biological agent in organisms that helps to form amino acids, which are the basic building blocks of proteins. It also involved in the natural decomposition of plant and animal materials. When present in higher concentrations, ammonia gas is hazardous to workers and the public.
WHAT IS AMMONIA GAS USED FOR?
Many industries make use of ammonia gas for several applications:
- Cleaning chemicals
- Agricultural fertilizer
- Refrigerant gas that has in large part replaced chlorofluorocarbons (CFCs)
- Water purification
- Manufacturing plastics and other chemicals
- A building block for pharmaceuticals
AMMONIA GAS PROPERTIES

According to the National Institute for Occupational Safety and Health, ammonia has boiling temperature of -33°C (-28°F) — ammonia is a gas a room temperature. While it is lighter than air, a release of pressurized ammonia gas can collect at ground-level until the aerosol cloud becomes diluted and begins to rise. Dilute ammonia gas that has left the cloud / vapor phase will generally not collect in low-lying areas such as heavier-than-air gases such as hydrogen sulfide (H2S).
Ammonia gas is very hydrophilic, meaning it is water-loving. When stored as a gas or compressed liquid without presence of water moisture, ammonia is referred to as anhydrous ammonia.
IMPACT ON SAFETY
Upon release to the environment, ammonia gas is very quick to attach itself to moisture, such as found in a person’s eyes, mouth, throat, lungs and on his or her skin. Ammonia is very caustic, forming ammonium hydroxide that has a higher pH than water and can damage respiratory systems, disrupt vision and irritate or burn skin upon contact. The caustic action of ammonium hydroxide damages cell membranes, causing more liquid to be released that further interacts with ammonia gas, perpetuating the effects on the human body.
ODOR THRESHOLD OF AMMONIA GAS

Although not every organization agrees on the perceptible threshold, OSHA estimates that people begins to smell ammonia gas ranging from 5 to 50 ppm. Experience in the industry indicates that workers consistently subjected to weak ammonia levels may become somewhat desensitized to its presence.
AMMONIA GAS EXPOSURE
According to Alberta Agriculture and Forestry, nose and throat irritation can result from ammonia exposure ranging from 24–50 parts per million (ppm) after ten minutes of exposure. With a higher ammonia concentration ranging from 72–134 ppm, the same irritation can occur in half the time. For a concentration of 700 ppm, immediate and severe irritation would likely occur. At a concentration of 5,000 ppm, respiratory spasms and rapid suffocation occurs. At 10,000 ppm, pulmonary edema and potentially fatal accumulation of liquid in the lungs would occur.
One thing common to almost every segment of the food and beverage industry is cold storage. Whether it is flash freezing in spiral or walk in freezers or simply sustained cold storage for finished products, cold storage units are used throughout the industry. This means one particular gas hazard comes along with them – ammonia (NH3).
Ammonia is the primary refrigerant in the commercial refrigeration equipment found in the food and beverage industry. Ammonia can leak and accumulate in areas around a freezer or cold storage unit and may be found at hazardous levels inside the unit itself.
Ammonia can present three major safety issues:
- Contamination of the products in cold storage
- Health risks to workers at certain concentrations
- Fire or explosion at certain concentrations
If you’re exposed to high concentrations of ammonia, you may notice immediate irritation and burning in your eyes, nose, throat, and respiratory tract. If you inhale high concentrations of ammonia, it could lead to burns to your mouth or nose, lung damage, blindness, or even death. Exposure to a concentration of just 300 PPM is considered to be immediately dangerous to life and health and may cause death within 30 minutes.
The impacts of leaking ammonia go beyond the toxic hazards it presents to workers. In high concentrations, ammonia is extremely combustible, with a lower explosive limit of 15% by volume or 150,000 PPM. While exposure to these concentrations is immediately deadly to humans, ammonia accumulating to these concentrations in unknown areas can present a greater hazard overall. At concentrations above 15%, ammonia combined with a source of ignition will create an explosion or fire that has catastrophic consequences.
Whether it is contaminating the product in storage or threatening a worker that may enter a particular unit, it’s critical to your safety that you understand the hazard presented by ammonia and monitor it accordingly. This means using a monitor with an appropriate measuring range for ammonia and combustible gases so you know exactly what precautions to take. Donning an SCBA to keep working when your personal ammonia monitor is over range may not keep you safe at all if ammonia reaches its LEL in the environment. Likewise, if your gas detector is only equipped with a combustible gas sensor, a dangerous level of ammonia may be present without ever registering a blip on the monitor.
Instead, if ammonia concentrations go beyond the range of your personal gas monitor, stop all hot work immediately, get a multi-gas monitor with a combustible gas sensor, and verify that the ammonia concentration is well below the explosive limit and at a level that allows you to continue working safely.
Don’t be caught out in the cold. Whether your choice for ammonia detection is a single gas monitor like the JXBS-3001-NH3
DETECTION OF AMMONIA GAS
As with all safety regulations, the safe work exposure limits for ammonia vary from region to region.
- In the United Kingdom, the Health and Safety Executive specifies safe exposure limits for ammonia gas as 25 ppm for an eight-hour time-weighted average (TWA) and 35 ppm for a 15-minute short-term exposure limit (STEL).
- In the United States, OSHA specifies the permissible exposure limit (PEL) for ammonia gas as 50 ppm for an eight-hour TWA, allowing a higher limit of 100 ppm for a shorter four-hour TWA.
The recommended exposure limit (REL) specified by the National Institute for Occupational Safety and Health (NIOSH) is 25 ppm for an eight-hour TWA. NIOSH specifies the immediately dangerous to life or health concentration (IDLH) at 500 ppm.
BLACKLINE SAFETY G7 WIRELESS GAS AND LONE WORKER MONITORING
While ammonia gas has a broad range of uses, from a refrigerant and fertilizer to being a raw material for many processes, it's hazardous to the health of nearby workers and the public. Personal gas detection with an ammonia sensor is an invaluable method for businesses to help personnel keep an eye on safe work environments and the potential for ammonia gas exposure.
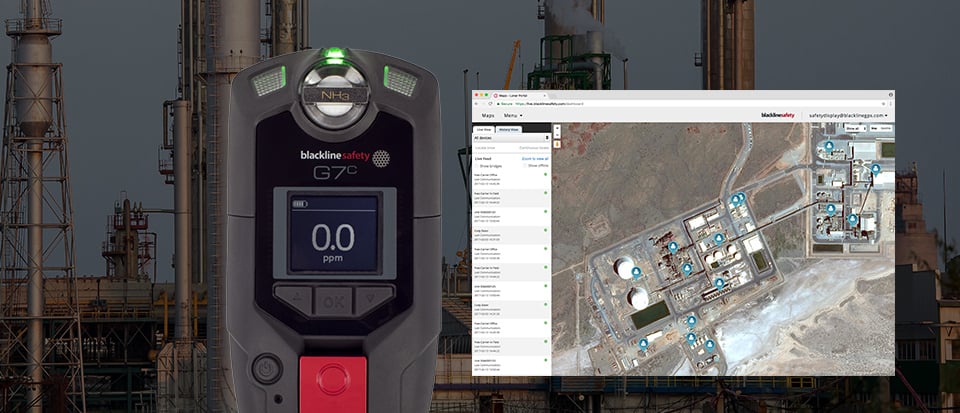
Wireless gas detection and communications solves the challenge of triggering an emergency response when a worker has been exposed to ammonia gas and cannot self-rescue. Blackline Safety developed its G7 wireless gas detection and lone worker monitors to solve this exact problem — to empower a real-time emergency response for when seconds count. G7 features an ammonia gas sensor option that alerts live monitoring personnel when an employee needs help, directing responders to the employee's exact location. Best of all, G7 safety monitoring technology and cloud-based monitoring portal are a turnkey solution that don't rely on facility Wi-Fi networks or power to operate. As a standalone system, no smartphones or Bluetooth connections are required.

![[JXBS-3001-NH3-LORA] LoRaWAN NH3 Ammonia Gas sensor](/web/image/product.template/48318/image_512/%5BJXBS-3001-NH3-LORA%5D%20LoRaWAN%20NH3%20Ammonia%20Gas%20sensor?unique=539c3a4)
![[JXCT-3001-NH3-1-100P] Wall Mounted NH3 Gas Sensor 3 in 1 Ammonia Gas Detector](/web/image/product.template/48311/image_512/%5BJXCT-3001-NH3-1-100P%5D%20Wall%20Mounted%20NH3%20Gas%20Sensor%203%20in%201%20Ammonia%20Gas%20Detector?unique=539c3a4)
![[JXBS-3001-BG-1] Water Ammonia Nitrogen ion Sensor](/web/image/product.template/48285/image_512/%5BJXBS-3001-BG-1%5D%20Water%20Ammonia%20Nitrogen%20ion%20Sensor?unique=539c3a4)